Opportunistic Lab Intern:
“While you’re all debating if it’s half full or half empty I drank it. Now it’s empty.”
Engineer: The glass is twice as big as it needs to be.
Bad Engineer: The glass is twice as big as it needs to be.
Good Engineer: The glass is 66% full with a 25% safety margin.This glass has a safety factor of 2
Re: good engineer: this is the thing that frustrates me amount marketing/labeling for travel mugs or cookware; the listed capacity is the absolute brim capacity not the practical capacity. Want to put 16 oz in a 16 oz mug you’re gonna have to sip 3 ounces out first in order to put the lid on. Want to serve 2 qt soup? Gotta use the 3 qt pot.
Yes!! So unbelievably annoying. Okay. Thank you. The total volume of this cylinder is 473ml. What the fuck can I use this for?! What I want you to tell me: total volume and total practical volume. Dumb af
Real engineer: it’s full. Approximately 50% water, and the rest air.
“The glass was built to the wrong specifications”
Glass functioning as intended. Any deficiencies that arise are due to the failure of the customer to provide appropriate design parameters.
Backyard tinkerer and wannabe Engineer: I’ll just use this glass jar I used to drain some gas as the thing to drink my water now … this is water right?
Not if you need to stir it.
Realist: who’s cleaning all these glasses?
Fucking real
The beaker is always full, when it’s half full of water, it is also at the same time half full of air. THE GLASS IS ALWAYS FULL
for some reason that was never acceptable answer when I gave it. i think they were just jealous they didn’t think of it.
Hopefully, otherwise it may end bad: https://what-if.xkcd.com/6/
But what if I pour vacuum into the beaker?
Then it would be full of dust bunnies
Full of vacuum
I’d like a bagel with everything
Everything is busy at the moment, but i heard Satan is free.
The beaker is always full, when it’s half full of water, it is also at the same time half full of air. THE GLASS IS ALWAYS FULL
Pedant
technically correct, which is the best kind of correct
My mother in-law is a lab scientist. She says this is accurate.
You don’t even know the half of it
I think they do know half of it.

Ah, it’s this time of the year again.
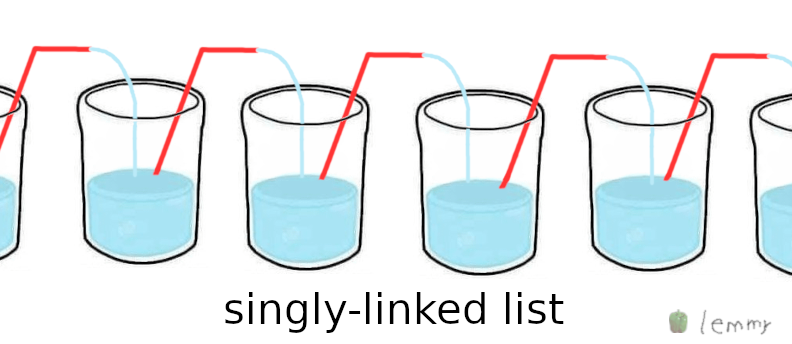 (version 3)
(version 3)Does this work?
If you looped it or created a doubly linked list what would happen?
Either perpetual motion, or a very wet desk, no in-between
This particular version wouldn’t work because the exit point is not lower than the entry point so after a possible initial splash from the first glass the outside air would rush in from the top of the straw and thus push down the water to its own level again…
So sadly no singly linked lists without stairs!
That makes sense
I knew there had to be a catch
My lab is pretty easy to guess, it’s either 18 MΩ water, 100% EtOH, or 16M HNO3. 66% chance it’s not acutely dangerous, not bad for a lab!
Sticky, Silky, and Danger Syrup! Sounds like a cool lab.
16M HNO3? Wouldn’t that be fuming?
It’s technically like 15.7M , it’s the highest concentration you can get before you hit fuming (~70wt% iirc). Although anything you do with it after makes it fume like crazy anyway.
Scientist Russian Roulette: Drink the mystery breaker. They all have water, except for one that’s hydroflouric acid.
Ahh found that label!:
99.985% Pure.
Nitrogen (N₂): 39%
Oxygen (O₂): 10.5%
Argon (Ar): 0.465%
Carbon dioxide (CO₂): 0.02%
Water (H₂O): 50%
I thought the half full, half empty thing. Was about the flow of water. If you’re emptying the glass, at some point the glass will be half empty. If you fill the glass, at one point the glass will be half full.
100% Full with 50% volume occupied by Dihydrogen monoxide molecules and 50% volume occupied by a mixture of molecules in gas form, colloquially refer as “air”, which contains, according to the statistical data recorded by analyzing the gas molecules in the air in the Earth’s Atmosphere, 78.08% nitrogen, 20.95% oxygen, 0.93% argon, 0.04% carbon dioxide, and small amounts of other trace gases.
🤓
(I have no idea what I’m saying lol)
Scientific paper writer
After a long romp, a fairly new g/f went into my kitchen, grabbed a 1 gal bottle of white vinegar from the fridge, poured herself a glass and tried to chugged it while I was still in bed recovering. -She had the nerve to think I tried to poison her (for half a minute)!
Read and use labels. lol
Why do you keep vinegar in the fridge? I keep the gallon jug in a cupboard and smaller container just on the counter
Why do you keep vinegar in the fridge?
Imagine the trouble if it rots!
When does that happen? I’ve had vinegar for years…
Loool, and do you keep sodium cyanide in your spice cabinet too?
Probably a good idea for the winter since fresh apricot seeds would be scarcer. j/k
I like to call the sodium cyanide in my lab Almond Joy
How much vinegar do you use???
It’s good for toilet bowl cleaner, weed killer (including poison ivy), wiping down a large cutting board (not used for meat), fruit and veg wash, descaling / removing water marks, rainbow stains or chrome / nickel residue in pans, it softens fabrics if added to laundry, is used for mayonnaise, salad dressings, sticky rice, deodorizing, and combined with baking soda has been the only thing that worked for a clogged drain. It’s also cheap in the gallon size and practically free for people on SNAP. (a lot)
That’s a lot of good reasons for keeping a large quantity of vinegar, but I think we’re still mystified as to why you keep it in the fridge
I don’t anymore, but… I liked it cold for some applications like canned spinach (cools that steaming pile of mushy green down). If I were to make things like mayonnaise with it, it keeps the ingredients in the safe temperature zone or gets them there faster.
Huh, yeah that makes a ton of sense. I’m gonna have to find a recipe for spinach canned with vinegar, that sounds fire
Oh, I use cleaner vinegar for that, it’s cheaper, but I don’t think you’re supposed to eat that. I guess just getting a jerrycan of edible vinegar makes sense.
Engineer: the glass is twice as big as in needs to be.